During the clinical management of cancer, the reporting of symptoms and side-effects is essential to the success of the treatment.
However, given the length of time between each follow-up visit, some patients prefer to discontinue treatment in the event of side effects while awaiting medical advice, and others may not remember the side effects at the time of the visit.
In addition, the description of symptoms may be imprecise for the doctor, or may be deliberately understated for fear of a change in prescription.
This is why increasing the frequency and quality of patient/doctor communications is a major challenge.
Establishments initially set up punctual telephone exchange systems, before the arrival of new digital remote monitoring tools. Now that there is sufficient evidence that these tools are effective, the European Society of Oncology (ESMO) published its first recommendations for their use in April 2022.
New recommendations to improve patient monitoring
These recommendations follow on from the results of eight clinical studies, including the French CAPRI study, which looked at the benefits of using digital tools to report the various symptoms experienced by patients.
These studies were published over several years and involved thousands of patients suffering from different types of cancer. What they had in common was that patients were asked to report the various symptoms they experienced during their treatment (anxiety, diarrhoea, nausea, etc.), along with their incidence and severity. The information was analysed by the healthcare teams, so that a diagnosis could be made as quickly as possible and appropriate action taken.
According to Prof. Fabrice Denis (Institut Inter-Regional de Cancérologie Jean Bernard in Le Mans), these studies have shown the “positive impact of remote monitoring in oncology in terms of improving overall survival, quality of life and compliance with treatment, as well as reducing symptoms, stopping treatment due to side-effects and emergency hospitalisation“.
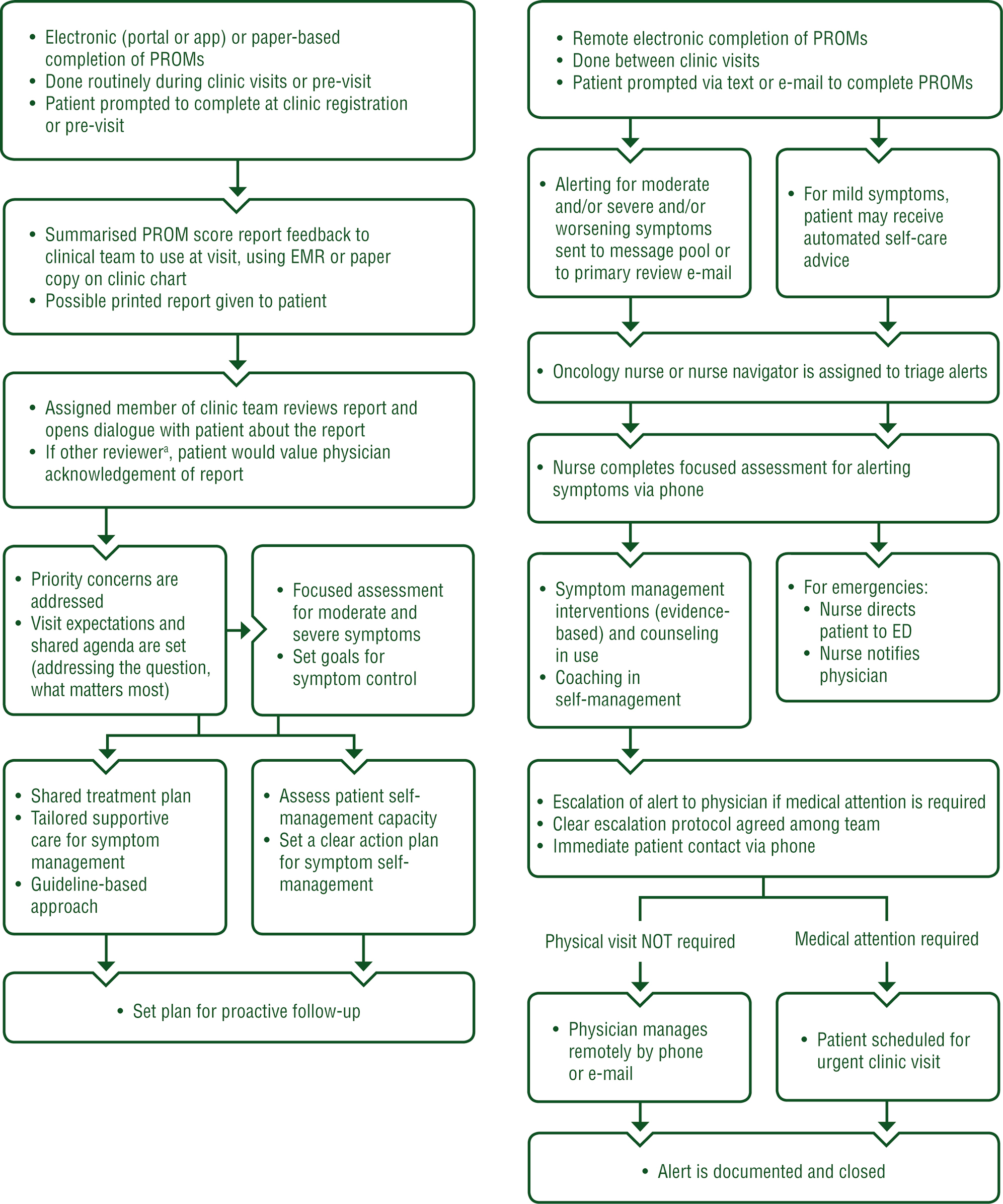
The recommendations define the criteria for selecting tools (class IIa CE marking, monitoring of all types of cancer, compliance with the RGPD, etc.), and propose the protocol illustrated below:
Recognition of the effectiveness of these digital tools and the definition of selection criteria will accelerate the spread of these practices, to the benefit of both patients and carers.
Links: